Multi-target cell and gene therapiesfor unmet medical needs
Aurealis 4-in-1 multi-target cell and gene therapy platform is the game-changing solution for complex multi-factorial diseases.
Discover how our technology is re-defining the treatment of chronic wounds (diabetic foot ulcers, venous leg ulcers), cancers (ovarian, peritoneal, bladder), and beyond.
THE TEAM, THE SCIENCE & the outcome
THE AUREALIS TEAM
At Aurealis Therapeutics, we are passionate about advancing science to make a difference for patients suffering from chronic wounds, deadly cancers, inflammatory diseases.
Meet the Management Team, the Scientific Advisors, the Board of Directors, and the Aurealis Therapeutics Team Members who make it happen.
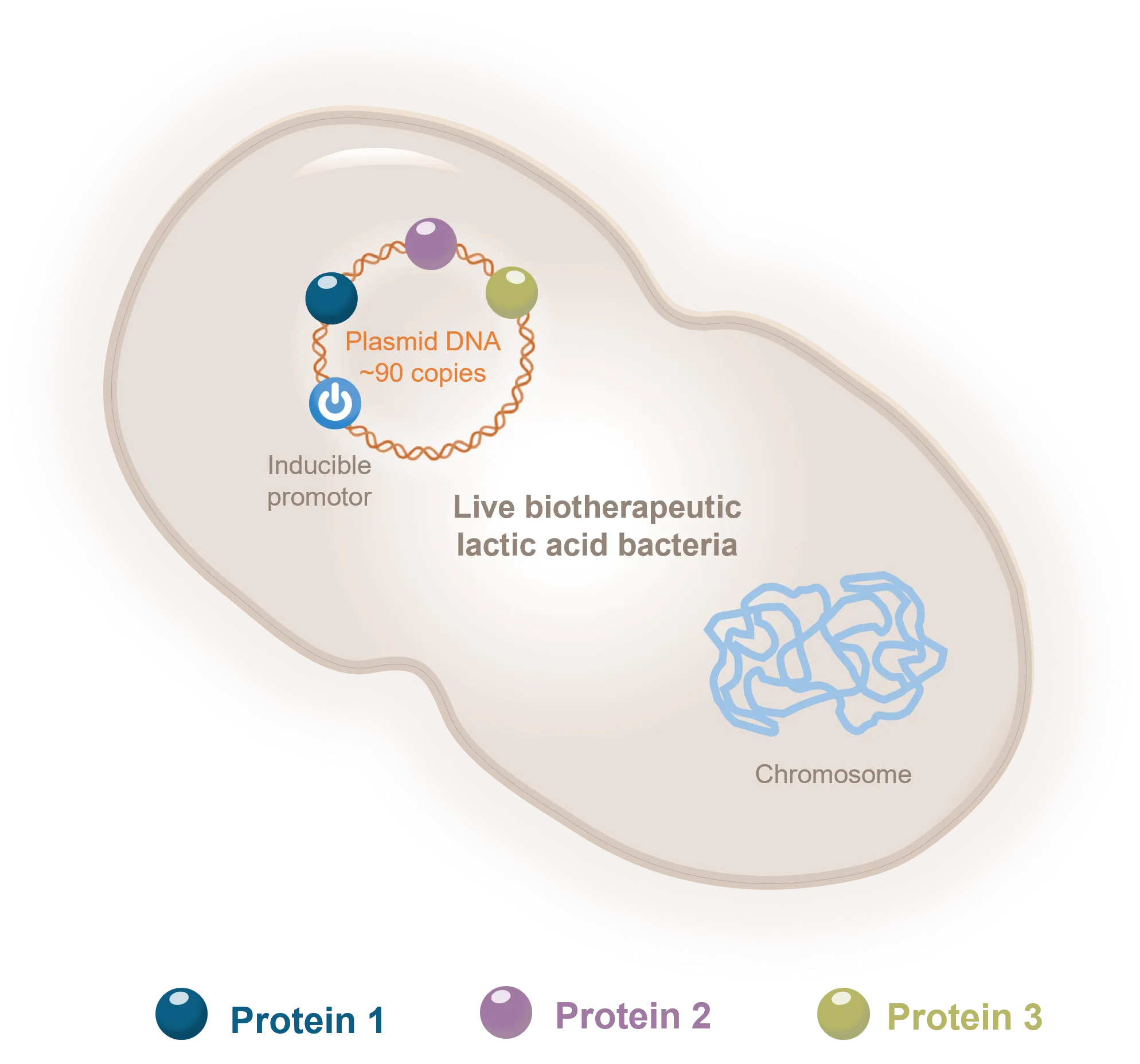
THE SCIENCE
Our team takes pride in mastering the most advanced cell and gene therapy science, and making work in real life, from the lab to the clinic.
Discover how our multi-target cell and gene therapy platform allows to modulate tissue microenvironment and tackle complex multi-factorial diseases.
THE OUTCOME
Generating pre-clinical, clinical and health-economic evidence is how we want to make a difference. Seeing non-healing wounds heal again, seeing how cancer deaths can be prevented, and ultimately improving patient outcome – this is what matters the most to us.
Interview with Hanna-Riikka Kärkkäinen Head of Quality and Regulatory Affairs at Aurealis Therapeutics | Professional career, AUP-16 PRIME designation, FDA Advice & more
What was the journey that took Hanna-Riikka Kärkkäinen to her current role as Head of Quality and Regulatory Affairs? What does having the PRIME designation for AUP-16 mean? What was...
Half of the Patients Successfully Recruited and Randomized in Aurealis Therapeutics DIAMEND AUP-16 Phase-2 Study
Zug, Switzerland and Kuopio, Finland, March 11, 2024. Aurealis Therapeutics, a Swiss-Nordic clinical-stage company developing scalable and low COGS multi-target cell and gene therapies, announced today the successful recruitment and...
Aurealis Therapeutics Receives Priority Medicines (PRIME) Designation from EMA for Enhanced Regulatory Support and Accelerated Assessment of AUP-16 in Non-healing Diabetic Foot Ulcers
Priority medicines (PRIME) is a scheme run by the European Medicines Agency (EMA) to enhance support for the development of medicines that target an unmet medical need. The scheme is...
Interview with Haritha Samaranayake CMO of Aurealis Therapeutics | Professional journey, AUP-16 clinical trials in DFU & development plans for oncology
Watch full interview on YouTube What was the journey that took Haritha Samaranayake to his current role as CMO? What are the current advances in the clinical trials of Diabetic...
Aurealis Therapeutics Partner Xbiome Receives IND Approval from China’s National Medical Products Administration for the Phase 2 Study with AUP-16 in Diabetic Foot Ulcers
Xbiome announced that its Phase 2 study with AUP-16 (AUP1602-C) in diabetic foot ulcer has officially obtained IND approval from the National Medical Products Administration (NMPA) and the study can...
AUP-16: More cost-effective than the current Standard Of Care at healing Diabetic Foot Ulcers revealed at ISPOR Europe 2023
Aurealis Therapeutics, a Swiss-Finnish clinical-stage synthetic biology company developing bacteria-based multi-target cell and gene therapies for chronic wounds and cancer, participated in ISPOR Europe Health Economics and Market Access conference...

